
|
GENTAUR EUROPE BELGIUM1 tel +32 2 732 5688 fax +32 2 732 4414 [email protected] Av. de l' Armée 68 B-1040 Brussels France tel 01 43 25 01 50 fax 01 43 25 01 60 9, rue Lagrange 75005 Paris Italy tel 02 36 00 65 93 fax +32 16 50 90 45 20135 Milano Germany tel +32 16 58 90 45 fax +32 16 50 90 45 Forckenbeckstraße 6 D-52074 Aachen Japan tel +81 78 386 0860 fax +81 78 306 0296 Minaatojimaminami-manchi Chuo-ku, Kobe 065-0047 |
Stabil-P.A.C. NV10 Stabilises Fusion Proteins after Tag Cleavage
The expression of recombinant proteins with fusion
partners can result in enhanced expression yields, increased
solubility and an improved purification route. Subsequent site specific
proteolysis is often required to remove these
fusion partners after purification in order to produce native protein. However,
cleavage of fusion tags which have
been added to enhance solubility frequently results in protein aggregation and
loss of yield. The presence of NV10
in cleavage buffers greatly improves protein solubility while maintaining high
cleavage efficiency. NV10 can be
readily removed from cleaved protein solutions by ion exchange or affinity
chromatography, but its compatibility with
many downstream applications often allows the user to retain NV10 in solution
with target protein, maintaining
protein solubility and stability.
PROTOCOL
Aggregation and stability are very protein specific, but a general protocol is given below.
1. Determine the starting protein
concentration (using eg. Bradford assay, BCA assay,
absorbance at 280nm).
2. Typically a fivefold excess, by mass, of
NV10 will protect the target fusion protein. For example, use 5 mg/ml
NV10 for 1
mg/ml protein.
3. Each Stabil-P.A.C. tube contains
1.25 mg NV10 as a lyophilised powder.
4. Add the protein solution to NV10
in Stabil-P.A.C. tubes to get the desired concentration, or make up a
10 mg/ml
stock of NV10 (4 X stock) by adding 125 μL of buffer or distilled water to each
Stabil-P.A.C. tube
and add
this stock to the protein cleavage solution before adding the protease.
5. Alternatively, make up the
cleavage buffer with the desired NV10 concentration, and use
PD10 desalting
columns to buffer exchange
the target fusion protein into cleavage buffer containing NV10.
6. Continue with tag cleavage
according to the standard protocol.
7. NV10 associates with the protein
in solution and protects the cleaved native protein from aggregation and
instability.
8. NV10 1X stock solution can be
stored for 1 week at 4 oC or for longer term at -20 oC.
Troubleshooting
• If the protein shows signs of aggregation or heavy losses
on cleavage then the relative NV10 concentration
can be increased, ie increase NV10
concentration and / or reduce protein concentration.
• Alternatively, a lower NV10 to protein ratio can be used
with proteins that have no history of aggregation.
EXAMPLE : Use of NV10 in Fusion Protein Stabilisation
A kinase protein was prepared with maltose binding protein as a fusion partner
(k-MBP) to enable facile purification
and high solubility. Cleavage of the fusion tag using Factor Xa routinely
resulted in heavy aggregation and
associated low yields of the native kinase. 1 mg/ml k-MBP was prepared in
cleavage buffer (20 mM Tris.HCl,
75 mM NaCl, 1 mM CaCl2, pH 6.5) containing an increasing concentration of NV10,
and then the protease
Factor Xa was added to initiate cleavage of the MBP tag. The solutions were
incubated at room temperature for
4 hours, and aggregation was monitored at intervals of 1 hour. The absorbance at
492 nm was used as a
measurement of aggregation.
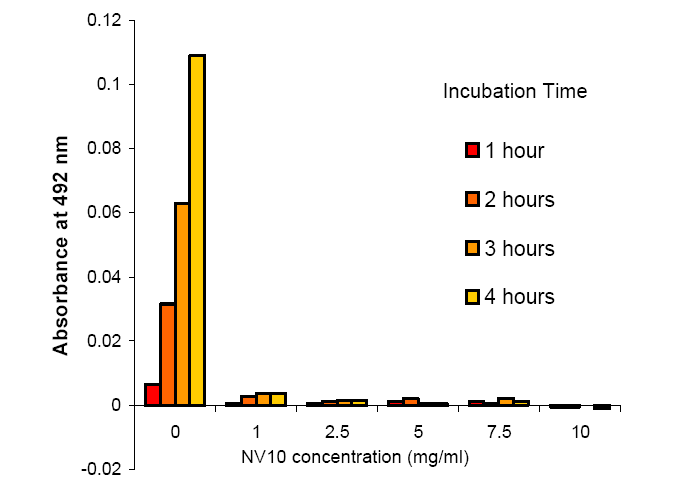
![]()
Cleavage of the fusion tag from k-MBP
using a standard cleavage buffer results in severe aggregation and loss of
protein yield as indicated by relatively high absorbance values at 492nm. The
presence of NV10 in the cleavage
buffer significantly reduces aggregation, and this is virtually eliminated at a
ratio of 5 mg/ml NV10 : 1 mg/ml protein
or above.
Summary
NV10 can
protect proteins from aggregation and loss of yield after fusion tag cleavage.

