|
GENTAUR EUROPE BELGIUM1 tel +32 2 732 5688 fax +32 2 732 4414 [email protected] Av. de l' Armée 68 B-1040 Brussels France tel 01 43 25 01 50 fax 01 43 25 01 60 9, rue Lagrange 75005 Paris Italy tel 02 36 00 65 93 fax +32 16 50 90 45 20135 Milano Germany tel +32 16 58 90 45 fax +32 16 50 90 45 Forckenbeckstraße 6 D-52074 Aachen Japan tel +81 78 386 0860 fax +81 78 306 0296 Minaatojimaminami-manchi Chuo-ku, Kobe 065-0047 |
J Clin Pathol 2000; 53:518-524
© 2000 Journal of Clinical
Pathology
Immunohistowax processing, a new fixation and embedding method for light microscopy, which preserves antigen immunoreactivity and morphological structures: visualisation of dendritic cells in peripheral organs
1 The Département de Biologie Moléculaire,
Université Libre de Bruxelles, B-1640 Rhode-Saint-Genése, Belgium
2 Immunex Corporation, Seattle, Washington 98101, USA
3 Cantab Pharmaceuticals Research LDT, Cambridge CB4 OWG, UK
Accepted for publication January 20, 2000 .
|
|
Abstract |
|---|
Aims—To describe a new fixation and embedding method for
tissue samples, immunohistowax processing, which preserves both
morphology and antigen immunoreactivity, and to use this technique to
investigate the role of dendritic cells in the immune response in
peripheral tissues.
Methods—This technique was used to stain a population of specialised antigen presenting cells (dendritic cells) that have the unique capacity to sensitise naive T cells, and therefore to induce primary immune responses. The numbers of dendritic cells in peripheral organs of mice either untreated or injected with live Escherichia coli were compared.
Results—Numbers of dendritic cells were greatly decreased in heart, kidney, and intestine after the inoculation of bacteria. The numbers of dendritic cells in the lung did not seem to be affected by the injection of E coli. However, staining of lung sections revealed that some monocyte like cells acquired morphological and phenotypic features of dendritic cells, and migrated into blood vessels.
Conclusions—These observations suggest that the injection of bacteria induces the activation of dendritic cells in peripheral organs, where they play the role of sentinels, and/or their movement into lymphoid organs, where T cell priming is likely to occur.
Key Words: dendritic cell • Escherichia coli • immunohistochemistry
|
|
Introduction |
|---|
The immune response is the result of multiple interactions between
discrete cell populations of the immune system. Specialised cells
(antigen presenting cells (APCs)) present the antigen, in the form of
peptides in the groove of major histocompatibility complex (MHC)
molecules, to helper T cells, which in turn activate the
differentiation of effector cells, such as B cells or cytotoxic
effector cells. APCs are widely distributed in the body, in lymphoid
and non-lymphoid organs,1 whereas T cells
recirculate through the lymphoid organs, where they are located in
discrete sites. There is evidence that T cell priming occurs in the T
cell zones of lymphoid organs.2,3
Therefore, the first step of the immune response is likely to involve
the migration of APCs and their redistribution into T cell areas.
The population of APCs is heterogeneous and includes dendritic cells, B cells, and macrophages. Among these cells, dendritic cells appear to have the unique capacity to sensitise naive T cells and are the APCs of the primary immune response.
The aim of our study was to analyse the movement of dendritic
cells in peripheral solid organs of mice injected with Gram negative
bacteria. To achieve this goal, a new immunohistochemistry processing
(immunohistowax processing) method, based on a proprietary fixation
and embedding medium, was developed and was shown to preserve both
morphology and antigen immunoreactivity. In this processing, protein
denaturation in the sample is minimised by the combined use of an
aldehyde free zinc salt solution and a new embedding wax
(Immunohistowax), which is liquid at low temperature (37°C). This
approach allowed us to identify dendritic cells by morphological as
well as phenotypic criteria. Our data show that most dendritic cells
disappear from kidney, heart, and intestine after the injection of
bacteria, whereas some lung dendritic cells become activated under
the same conditions.
|
|
Materials and methods |
|---|
ANIMALS
Female Balb/c and C57BL/6 mice were purchased from IFFA-CREDO
(Brussels, Belgium) and maintained in our pathogen free facility.
escherichia coli inoculation
The K504 E coli strain was kindly provided by Dr E Van Driessche
(Laboratorium voor Chemie der Proteinen, Vrije Universiteit
Brussel, Belgium). Bacteria were grown in LB medium overnight at
37°C. Bacteria were pelleted by centrifugation at 3000
xg for 10 minutes, washed twice in
sterile phosphate buffered saline (PBS) and resuspended at 5
x 108 bacteria/ml in PBS.
A standard of absorbencies based on known colony forming units
(CFU) was used to calculate the inoculum concentration. Mice received
an intravenous injection of 200 µl of E coli suspension. An
E coli dose of 108 CFU was used in all experiments.
IMMUNOHISTOWAX PROCESSING
The tissue sample was fixed in a formaldehyde free zinc fixative
(Immunohistofix; Intertiles, Brussels, Belgium) for three days at
4°C. Sample thickness did not exceed 5 mm to allow optimal
infiltration of fixative and dehydrating agents. Dehydration was
performed according to two different protocols: the samples were
either dehydrated in a graded series of ethanol baths: 30%, 50%, 70%,
90%, and 100% for 30 minutes each at room temperature, or samples
were dehydrated in 100% acetone for six hours. The first protocol
seems to preserve tissue morphology more effectively, whereas the
second possibly preserved some antigens more efficiently.
Infiltration was performed at 37°C by means of three baths of
Immunohistowax for 20 minutes each. Tissue specimens were then
embedded in Immunohistowax and mounted on wooden blocks. Blocks were
stored at 4°C for at least one night before sectioning, and could be
kept for up to six months at room temperature. Blocks can become
difficult to cut at temperatures above 22°C, and were therefore kept
at 4°C and cut promptly. Sections of 3–5 µm were performed with a
sliding microtome and individual sections were transferred directly
using thin grids on a drop of water on gelatin precoated slides.
Floating on a water bath is unsuitable because of the slight
hydrophilicity of the wax. Slides were air dried at room temperature
and stored at room temperature for up to several months.
IMMUNOSTAINING
Immunohistowax processed sections were dewaxed in acetone for one to
five minutes and transferred to PBS. Immunohistochemical staining was
performed as follows.
Inhibition of endogenous peroxidases
If peroxidase was used for visualisation, the slides were first
treated with 3% H2O2 in PBS for 30 to 60 minutes to block
endogenous peroxidase; the use of methanol was avoided because it
might be detrimental for certain antigens, such as T cell markers.
Saturation step
We commonly use the blocking reagent (catalogue number, 1096176) from
Boehringer (Brussels, Belgium; 1% in PBS (PBS-BR)) to saturate
non-specific reaction sites because it gives a lower background than
bovine serum albumin, horse serum, or goat serum. Sections were
incubated in PBS-BR at room temperature for 30 minutes.
Single staining
Sections were washed in PBS and incubated with antibodies (5–25
µg/ml) for one to three hours at room temperature or overnight at 4°C
in PBS-BR. Primary monoclonal antibodies gave better results when
coupled to biotin or fluorescein isothiocyanate (FITC). Biotinylated
antibodies were visualised with ABC kits from Vector Laboratories
(Burlingame, California, USA; 1/100 in PBS-BR) for 30 minutes at room
temperature. Peroxidase was revealed using either diaminobenzidine
substrates with or without metal enhancer (Sigma, Bornem, Belgium),
TMB, Vector SG Substrate Kit, Vector VIP Substrate Kit (Vector), or
AEC (Sigma). Substrate kits from Vector Laboratories for alkaline
phosphatase were used according to the manufacturer's
recommendations. FITC conjugated antibodies were visualised by
incubation for 30 minutes with anti-FITC alkaline phosphatase or
peroxidase Fab fragment (Boehringer), diluted at 1/500 in PBS-BR.
Counterstaining
Single immunostained sections were counterstained with haematoxylin
or methyl green depending on the substrate colour.
When double or triple staining was performed with biotinylated antibodies, excess biotin from the first antibody was blocked with the Vector blocking kit. In the case of multiple peroxidase stainings, enzymatic activity linked to the first antibody was neutralised by incubating sections in H2O2 (3% in PBS) for 20–30 minutes.
Mounting
Slides were mounted in Aquatex (Merck, Overijse, Belgium) or
Polymount (Polysciences, Warrington, USA) depending on the solubility
of substrates.
|
|
Results |
|---|
IMMUNOHISTOWAX PROCESSING
Morphological observations require thin tissue sections, which are
best obtained after the infiltration and embedding of specimens with
wax or plastic. However, because waxes are poorly soluble in water,
their use requires pretreatment with fixatives and dehydrating
solvents, which is known to affect protein structure. In addition,
fixation and/or embedding can also affect the immunodetection of many
cellular markers as a result of protein denaturation caused by
chemical and/or thermal injury.4–6
Although we have developed a novel wax that is liquid at near
physiological temperature (37°C), its poor solubility in water
required tissue dehydration before embedding. Dehydration was
performed with acetone or ethanol, which were fully miscible in the
liquid wax. Unfortunately, and as expected from previous reports,7
the dehydration step affected the staining of many antigens.
The data in table 1![]() show that dehydration prevented the staining of CD3, CD4, and CD8
antigens. By contrast, other antigens, such as MHC class II, CD11c,
B220, and Mac-1 were not altered by dehydration with one bath of 100%
acetone for six hours.
show that dehydration prevented the staining of CD3, CD4, and CD8
antigens. By contrast, other antigens, such as MHC class II, CD11c,
B220, and Mac-1 were not altered by dehydration with one bath of 100%
acetone for six hours.
|
Because exposure to organic solvents before embedding could not be
avoided, we attempted to protect antigenic structures from chemical
denaturation by increasing protein stability before fixation.
Engineered metal chelation in proteins has been used successfully to
stabilise proteins against denaturation.8,9
We choose Zn2+ as a metal ion, based on its known ability to
interact with at least four amino acids (primarily histidine,
and to a lesser extent, aspartic acid, glutamic acid, and cysteine)
through binding to nitrogen, oxygen, and sulphur atoms.10,11
Its thermodynamic properties have been shown to promote favourable
entropic effects, which enhance the stability of secondary protein
structure.12 Ligand motifs that can be used
for metal binding are His-XXX-His for an
![]() -helix,
His-X-His for a ß-strand,
and His-XX-His for a reverse type II ß-strand.13
In some cases, one or two histidine(s) might be replaced by aspartate
or cysteine. We analysed mouse protein sequences and found that
at least one motif was present in all sequences investigated, the
mean value being 3.5 motifs/sequence. Although structural data are
not available for most antigenic proteins, we assumed that a
sufficient number of these motifs was indeed able to bind a metal
ion.
-helix,
His-X-His for a ß-strand,
and His-XX-His for a reverse type II ß-strand.13
In some cases, one or two histidine(s) might be replaced by aspartate
or cysteine. We analysed mouse protein sequences and found that
at least one motif was present in all sequences investigated, the
mean value being 3.5 motifs/sequence. Although structural data are
not available for most antigenic proteins, we assumed that a
sufficient number of these motifs was indeed able to bind a metal
ion.
Tissue specimens were pretreated with a zinc fixative (Immunohistofix),
dehydrated, and embedded in Immunohistowax. We compared the
staining of several antigens on tissue samples, pretreated or not
with zinc fixative. The data in table 1![]() show that the staining of CD3, CD4, and CD8 antigens was preserved by
pretreatment with the zinc fixative. We further tested several
monoclonal or polyclonal antibodies, fusion proteins, lectins, and
enzymes for the staining of membrane or intracellular proteins,
carbohydrate residues, surface receptors, and apoptotic cells. The
results in table 2
show that the staining of CD3, CD4, and CD8 antigens was preserved by
pretreatment with the zinc fixative. We further tested several
monoclonal or polyclonal antibodies, fusion proteins, lectins, and
enzymes for the staining of membrane or intracellular proteins,
carbohydrate residues, surface receptors, and apoptotic cells. The
results in table 2![]() show that a large number of molecules expressed by T or B cells, NK
cells, macrophages, and dendritic cells could be detected on
Immunohistowax processed sections. In particular, determinants
expressed upon cell activation were stained using monoclonal
antibodies (specific for CD25, CD44, CD69, CD86, Ly-77, or DEC-205)
or a fusion protein (OX40 ligand–human IgG1). B cells specific for
the hapten arsonate were visualised on spleen sections using the
hapten coupled to bovine serum albumin (BSA), whereas T cells were
stained with a monoclonal antibody specific for their antigenic
receptor. Interleukin 2 (IL-2), IL-4, interferon
show that a large number of molecules expressed by T or B cells, NK
cells, macrophages, and dendritic cells could be detected on
Immunohistowax processed sections. In particular, determinants
expressed upon cell activation were stained using monoclonal
antibodies (specific for CD25, CD44, CD69, CD86, Ly-77, or DEC-205)
or a fusion protein (OX40 ligand–human IgG1). B cells specific for
the hapten arsonate were visualised on spleen sections using the
hapten coupled to bovine serum albumin (BSA), whereas T cells were
stained with a monoclonal antibody specific for their antigenic
receptor. Interleukin 2 (IL-2), IL-4, interferon
![]() (ifn-
(ifn-![]() ),
and IL-10 were detected in the cytoplasm of activated T cells in
situ, using double staining with antibodies to T cells and to
lymphokines. Using this processing method, we previously identified
apoptotic cells in tissues by double staining with antibodies to cell
surface markers and the TUNEL (TdT mediated dUTP nick end labelling)
reaction.25 It should be noted that, among
all presently tested antibodies, only two did not work with this
technique: 2C11 (hamster antimouse CD3) and GK1.5 (rat antimouse
CD4).
),
and IL-10 were detected in the cytoplasm of activated T cells in
situ, using double staining with antibodies to T cells and to
lymphokines. Using this processing method, we previously identified
apoptotic cells in tissues by double staining with antibodies to cell
surface markers and the TUNEL (TdT mediated dUTP nick end labelling)
reaction.25 It should be noted that, among
all presently tested antibodies, only two did not work with this
technique: 2C11 (hamster antimouse CD3) and GK1.5 (rat antimouse
CD4).
|
INJECTION OF GRAM NEGATIVE BACTERIA REDUCES THE NUMBER OF DENDRITIC
CELLS IN KIDNEY, HEART, AND INTESTINE
Dendritic cells are a trace population in most organs, usually
display dendrites, and express selected surface markers, such as MHC
class II molecules and/or CD11c. We stained sections of various
organs with CD11c or MHC class II specific monoclonal antibodies.
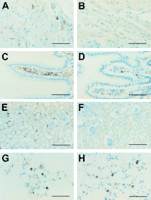
Dendritic cells in the kidney, heart, and intestine were found to be
negative for CD11c expression. In the heart, most class II positive
cells with a dendritic morphology (presumably dendritic cells) are
located in the pericardium (fig 1A![]() ),
whereas few cells were detected in the myocardium (not shown).
Dendritic cells in both sites decreased in numbers after the
injection of E coli (fig 1B
),
whereas few cells were detected in the myocardium (not shown).
Dendritic cells in both sites decreased in numbers after the
injection of E coli (fig 1B![]() and data not shown). In the intestine, class II positive cells were
detected in the connective tissue (lamina propria) of the villi (fig
1C
and data not shown). In the intestine, class II positive cells were
detected in the connective tissue (lamina propria) of the villi (fig
1C![]() ).
The injection of live bacteria resulted in the loss of most MHC class
II positive cells (fig 1D
).
The injection of live bacteria resulted in the loss of most MHC class
II positive cells (fig 1D![]() ).
As shown in fig 1E
).
As shown in fig 1E![]() ,
MHC class II positive cells with a typical dendritic morphology could
be found in the renal cortex around the glomeruli in the kidney of
PBS treated mice. Intravenous inoculation of E coli led to a
reduction in the number of MHC class II positive cells (fig 1F
,
MHC class II positive cells with a typical dendritic morphology could
be found in the renal cortex around the glomeruli in the kidney of
PBS treated mice. Intravenous inoculation of E coli led to a
reduction in the number of MHC class II positive cells (fig 1F![]() ).
).
|
INJECTION OF e coli induces phenotypic and morphological changes
in lung dendritic cells and their movement to blood vessels
By contrast, the numbers of CD11c positive cells in lung remained
unchanged after the inoculation of live E coli (compare fig 1G
and 1H![]()
![]() ).
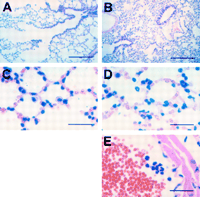
Staining of lung sections with haemalun-eosin clearly shows
constriction of the alveoli (fig 2A and B
).
Staining of lung sections with haemalun-eosin clearly shows
constriction of the alveoli (fig 2A and B![]() )
and infiltration of the lung parenchyma by neutrophils (fig 2D and E
)
and infiltration of the lung parenchyma by neutrophils (fig 2D and E![]() ),
starting one hour after the inoculation of live E coli. In
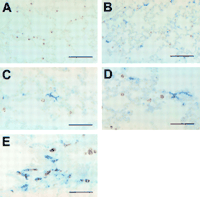
addition, immunostaining of sections revealed CD11c positive cells
with a globular shape within the alveoli (fig 3A
),
starting one hour after the inoculation of live E coli. In
addition, immunostaining of sections revealed CD11c positive cells
with a globular shape within the alveoli (fig 3A![]() ).
These cells did not express MHC class II molecules (fig 3A
).
These cells did not express MHC class II molecules (fig 3A![]() )
and could be alveolar macrophages or dendritic cells at a very
immature stage (see below). Surprisingly, some of these CD11c
positive cells (approximately 20–25%) increased in size, acquired a
dendritic morphology, and upregulated the expression of I-E molecules
(fig 3B
)
and could be alveolar macrophages or dendritic cells at a very
immature stage (see below). Surprisingly, some of these CD11c
positive cells (approximately 20–25%) increased in size, acquired a
dendritic morphology, and upregulated the expression of I-E molecules
(fig 3B![]() –E)
as early as one hour after the injection of live bacteria. Later on
(one to six hours after treatment), these dendritic like, CD11c
positive, MHC class II positive cells seem to migrate to the blood
vessels (fig 3C and D
–E)
as early as one hour after the injection of live bacteria. Later on
(one to six hours after treatment), these dendritic like, CD11c
positive, MHC class II positive cells seem to migrate to the blood
vessels (fig 3C and D![]() ).
).
|
|
|
|
Discussion |
|---|
Our report describes a new fixation and embedding technique, called
Immunohistowax processing, that permits immunostaining of a large
variety of antigens and preserves the morphology of all tissues
tested. To achieve minimal denaturation of proteins during
dehydration and embedding, the protein structures were stabilised by
pretreatment with an aldehyde free zinc fixative before dehydration
with ethanol or acetone. Tissue specimens were then embedded in an
inert wax at 37°C. This processing allowed antigen immunodetection
and morphological analysis of thin sections (3–5 µm).
The Immunohistowax processing technique allowed us to detect a trace population of APCs—cells of the dendritic family—in peripheral organs using phenotypic and morphological criteria.
It is generally believed that dendritic cells play the role of sentinels in the periphery, and upon the encounter of an appropriate signal (signal of danger?) are redistributed to T cell areas, where they probably prime T cells.26 The major role of dendritic cells in inducing primary immune responses correlates with some specialisation of function over time and space. In non-lymphoid tissues, dendritic cells are present in an immature state: well equipped to capture and process antigens but unable to sensitise T cells optimally. In secondary lymphoid organs, dendritic cells are mature; that is, they poorly capture and process proteins but have the capacity to prime naive T cells. The data presented here indicate that the inoculation of live bacteria induces the disappearance of most dendritic cells from the heart, kidney, and intestine. Their loss could be attributed to migration from these organs or death by apoptosis. We and others have shown previously that the injection of lipopolysaccharide or toxoplasma extracts provoked the migration of splenic dendritic cells from the marginal zone between the red and white pulp to the areas where T cells are located.27,28 These observations, together with those of Roake et al,29 suggest that dendritic cells that have encountered bacteria might migrate to T cell zones in lymph nodes. Experiments are under way to test whether new migrant cells, bearing microbial antigens, can be detected in draining lymph nodes.
In the pulmonary alveoli, some CD11c positive cells acquire a dendritic morphology, upregulate the expression of MHC class II molecules, and migrate into proximal capillaries. These observations are reminiscent of a report by Randolph et al,30 showing that monocytes differentiate into dendritic cells in vitro, after migration across the endothelium in the subluminal to lumenal direction, a phenomenon that is potentiated by an additional stimulus, such as lipopolysaccharide of zymosan particles. It is therefore tempting to speculate that, in the lung, MHC class II negative, CD11c positive monocytes are induced to differentiate into dendritic cells through crossendothelial migration and exposure to microorganisms. Additional work will be required to test this hypothesis.
In conclusion, Immunohistowax processing seems to allow optimal conservation of morphology and immunoreactivity. Indeed, almost all antigens tested were detected in Immunohistowax processed sections, including antigens that were not stained in paraffin wax embedded sections or cryosections. Of note, this method only requires standard equipment and does not rely on the antigen retrieval technique. We believe that Immunohistowax processing will be useful to identify in situ the cell populations that secrete various cytokines and to define more accurately the spatial and temporal organisation of the immune response.
|
|
Acknowledgments |
|---|
We thank Drs Ralph Steinman (Rockefeller Institute, New York, USA),
Gerry Klaus (National Institute for Medical Research, London, UK),
Hervé Bazin (Université Catholique de Louvain, Bruxelles, Belgium),
and John Shields (Cantab Pharmaceuticals, Cambridge, UK) for
providing useful reagents; G Dewasme, M Swaenepoel, F Tielemans, and
P Veirman for technical assistance; and D Nolan for editorial
assistance. The laboratory of animal physiology was supported by
grants of the Fonds National de la Recherche Scientifique
(FNRS)/Télévie, the Fonds de la Recherche Fondamentale Collective,
the European Commission (CEC TMR Network Contract FMRX-CT96–0053),
and the Belgian Programme on Interuniversity Poles of Attraction
initiated by the Belgian State, Prime Minister's Office, Science
Policy Programming. TDS, CDT, RM-L, and MM are supported by the FNRS.
|
|
References |
|---|
- Steinman RM, Pack M, Inaba K. Dendritic cells in the T-cell areas of lymphoid organs. Immunol Rev 1997;156:25–37.[Medline]
- Zinkernagel RM, Ehl S, Aichele P, et al. Antigen localization regulates immune responses in a dose- and time-dependent fashion: a geographical view of immune reactivity. Immunol Rev 1997;156:199–209.[Medline]
- Ingulli E, Mondino A, Khoruts A, et al. In vivo detection
of dendritic cell antigen presentation to CD4+ T cells. J Exp
Med 1997;185:2133–41.
[Abstract/Free Full Text] - Pudney J, Anderson D. Effects of fixation and paraffin embedding on the immunohistological detection of cell-associated HIV-1 by different monoclonal antibodies. J Histochem Cytochem 1995;41:857–62.
- van Stokkum IHM, Linsdell H, Hadden JM, et al. Temperature-induced changes in protein structures studied by Fourier transform infrared spectroscopy and global analysis. Biochemistry 1995;34:10508–18.[Medline]
- Narhi LO, Philo JS, Li T, et al. Induction of
 -helix
in the ß-sheet protein tumor necrosis factor-
-helix
in the ß-sheet protein tumor necrosis factor- :
thermal- and trifluoroethanol-induced denaturation at neutral pH.
Biochemistry
1996;35:11447–53.[Medline]
:
thermal- and trifluoroethanol-induced denaturation at neutral pH.
Biochemistry
1996;35:11447–53.[Medline]
- Thomas PD, Dill KA. Local and nonlocal interactions in globular
proteins and mechanisms of alcohol denaturation. Protein Sci 1993;2:2050–65.
[Abstract/Free Full Text] - Regan L, Clarke ND. A tetrahedral Zn(II)-binding site introduced into a designed protein. Biochemistry 1990;29:10878–83.[Medline]
- Kuroki R, Taniyama Y, Seko C, et al. Design and creation of a Ca2+ binding site in human lysozyme to enhance structural stability. Proc Natl Acad Sci U S A 1989;86:6903–7.[Abstract]
- Tainer JA, Roberts VA, Getzoff ED. Metal-binding site in proteins. Curr Opin Biotechnol 1991;2:582–91.[Medline]
- Tainer JA, Roberts VA, Getzoff ED. Protein metal-binding sites. Curr Opin Biotechnol 1992;3:378–87.[Medline]
- Christianson DW. Structural biology of zinc. Adv Protein Chem 1991;42:280–355.
- Arnold FH, Zhang J-H. Metal-mediated protein stabilization. Trends Biotechnol 1994;12:189–92.[Medline]
- Coulie PG, Uyttenhove C, Wauters P, et al. Identification of a murine monoclonal antibody specific for an allotypic determinant on mouse CD3. Eur J Immunol 1991;21:1703–9.[Medline]
- Peterman GM, Spencer C, Sperling AI, et al. Role of
gamma delta T cells in murine collagen-induced arthritis. J Immunol
1993;151:6546–58.
[Abstract/Free Full Text] - Payne J, Huber BT, Cannon NA, et al. Two monoclonal rat antibodies with specificity for the beta-chain variable region V beta 6 of the murine T-cell receptor. Proc Natl Acad Sci U S A 1998;85:7695–8.
- Lefebvre M, Voncenzotto C, Digneffe C, et al. Rat monoclonal antibodies against murine immunoglobulins. In: Bazin H, ed. Rat hybridomas and rat monoclonal antibodies. Boca Raton, Florida, USA: CRC Press Inc, 1988:231–4.
- Metlay JP, Witmer-Pack MD, Agger R, et al. The distinct leukocyte integrins of mouse spleen dendritic cells as identified with new hamster monoclonal antibodies. J Exp Med 1990;171:1753–71.[Abstract]
- Hasbold J, Johnson-Léger C, Atkins CJ, et al. Properties of mouse CD40: cellular distribution of CD40 and B cell activation by monoclonal anti-mouse CD40 antibodies. Eur J Immunol 1994;24:1835–42.[Medline]
- Inaba K, Steinman RM, Witmer-Pack M, et al. Identification of proliferating dendritic cell precursors in mouse blood. J Exp Med 1992;175:1157–67.[Abstract]
- Agger R, Witmer-Pack M, Romani N, et al. Two populations of splenic dendritic cells detected with M342, a new monoclonal to an intracellular antigen on interdigitating dendritic cells and some B lymphocytes. J Leukoc Biol 1992;52:34–42.[Abstract]
- Kraal G, Breel M, Jnase M, et al. Langerhans cells, veiled cells, and interdigitating cells in the mouse recognized by a monoclonal antibody. J Exp Med 1986;163:981–97.[Abstract]
- Hathcock KS, Lazlo G, Dickler HB, et al. Identification of an alternative CTLA4-ligand costimulatory for T cell activation. Science 1993;262:905–7.[Medline]
- Paineau J, Priestley C, Fabre J, et al. Effect of recombinant interferon gamma and interleukin-2 and of a monoclonal antibody against interferon gamma on the rat immune response against heart allografts. J Heart Lung Transplant 1991;10:424–30.[Medline]
- De Smedt T, Pajak B, Klaus GGB, et al. Antigen-specific
T lymphocytes regulate lipopolysaccharide-induced apoptosis of dendritic
cells in vivo. J Immunol 1998;161:4476–9.
[Abstract/Free Full Text] - Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature 1998;392:245–52.[Medline]
- De Smedt T, Pajak B, Muraille E, et al. Regulation of dendritic cell numbers and maturation by lipopolysaccharide in vivo. J Exp Med 1996;184:1413–24.[Abstract]
- Reis e Sousa C, Hieny S, Scharton-Kersten T, et al. In
vivo microbial stimulation induces rapid CD40 ligand-independent production
of interleukin 12 by dendritic cells and their redistribution to T cell
areas.
J Exp Med 1997;186:1819–29.
[Abstract/Free Full Text] - Roake JA, Rao AS, Morris PJ, et al. Dendritic cell loss from nonlymphoid tissues after systemic administration of lipopolysaccharide, tumor necrosis factor, and interleukin 1. J Exp Med 1995;181:2237–47.[Abstract]
- Randolph GJ, Beaulieu S, Lebecque S, et al.
Differentiation of monocytes into dendritic cells in a model of
transendothelial trafficking. Science 1998;282:480–3.
[Abstract/Free Full Text]