
|
GENTAUR EUROPE BELGIUM1 tel +32 2 732 5688 fax +32 2 732 4414 [email protected] Av. de l' Armée 68 B-1040 Brussels France tel 01 43 25 01 50 fax 01 43 25 01 60 9, rue Lagrange 75005 Paris Italy tel 02 36 00 65 93 fax +32 16 50 90 45 20135 Milano Germany tel +32 16 58 90 45 fax +32 16 50 90 45 Forckenbeckstraße 6 D-52074 Aachen Japan tel +81 78 386 0860 fax +81 78 306 0296 Minaatojimaminami-manchi Chuo-ku, Kobe 065-0047 |
Cardio MPO Reagent Kit 7601 1450 Euro
Cardio MPO Control Kit 8601
450 Euro
Cardio MPO Calibrator Kit 9601 542 Euro
Human Myeloperoxidase ELISA
CardioMPO™ ELISA based test kit for in vitro
diagnostic use. The CardioMPO™ Test measures myeloperoxidase (MPO) levels
in human plasma and is intended for use as an aid in evaluating patients
presenting with chest pain that are at risk for major adverse cardiac events
(MACE), including myocardial infarction, need for revascularization, or death.
The CardioMPO™ test kits are currently available and testing is available
through our CLIA-certified reference lab.

Heart Attack Diagnostic mpo elisa test kit has
received FDA approval for a
diagnostic blood test capable of identifying
patients who are in imminent danger of heart
attack or death. The test, called CardioMPO,
measures blood levels of the enzyme
myeloperoxidase (MPO). Cleveland Clinic
researchers found that elevated MPO levels in
patients complaining of chest pain can signal
a high risk of a heart attack or cardiac death
or an acute need for intervention.
The human MPO test kit has been developed for the
quantitative measurement of natural human MPO in plasma and culture medium.
Myeloperoxidase (MPO) is a glycoprotein with a alpha2beta2 heteromultimer
expressed in all cells of the myeloid linage. MPO is abundantly present in
azurophilic granules of polymorphonuclear neutrophils. It is an important enzyme
used during phagocytic lysis of engulfed foreign particles which takes part in
the defense of the organism through production of hypochlorous acid (HOCl), a
potent oxidant. MPO is rapidly released by activated polymorphonuclear
neutrophils. Involvement of MPO has been described in numerous diseases such as
atherosclerosis, lung cancer, Alzheimer's disease and multiple sclerosis.
Autoimmune antibodies to MPO are involved in Wegener’s disease. Since the
discovery of MPO deficiency, initially regarded as rare and restricted to
patients suffering from severe infections, MPO has attracted more clinical
attention.
The classical MPO assay is an enzymatic assay for activity of MPO. This
classical MPO assay is hampered by the presence of inhibitory compounds in many
tissue homogenates. In this type of assays spiking often gives unreliable
results. Human MPO ELISA is not influenced by inhibitors of the enzyme activity
due to the supplied buffer system.
Application:
The human MPO ELISA kit is intended for the quantitative measurement of the
human MPO in cell culture medium and plasma. In plasma samples MPO can be
measured accurately if plasma samples are diluted at least 10 times. Most
reliable results are obtained if Heparin plasma is used.
| CardioTACS™
In Situ Apoptosis Detection Kits |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||


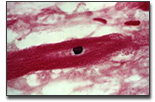 kit is based on DNA end-labeling using terminal deoxynucleotidyl
transferase (TdT) and a modified nucleotide that is subsequently
detected using our TACS Blue Label™
detection system. Trevigen has developed an exclusive apoptosis
grade TdT enzyme that coupled with the TACS Blue Label solution
provides labeling 20 to 50 times more sensitive than standard
diaminobenzidine (DAB) labeling methods. To ensure ease of data
interpretation when the numbers of apoptotic cells are low,
CardioTACS provides Nuclear Fast Red Counterstain which provides
superb contrast to the TACS Blue Label in apoptotic cells. In
addition, the kit includes TACS-Nuclease™
that is used to generate a positive control in your own sample.
The positive control provides an internal control for
permeabilization and labeling so optimization is nominal and the
data can be interpreted with confidence.
kit is based on DNA end-labeling using terminal deoxynucleotidyl
transferase (TdT) and a modified nucleotide that is subsequently
detected using our TACS Blue Label™
detection system. Trevigen has developed an exclusive apoptosis
grade TdT enzyme that coupled with the TACS Blue Label solution
provides labeling 20 to 50 times more sensitive than standard
diaminobenzidine (DAB) labeling methods. To ensure ease of data
interpretation when the numbers of apoptotic cells are low,
CardioTACS provides Nuclear Fast Red Counterstain which provides
superb contrast to the TACS Blue Label in apoptotic cells. In
addition, the kit includes TACS-Nuclease™
that is used to generate a positive control in your own sample.
The positive control provides an internal control for
permeabilization and labeling so optimization is nominal and the
data can be interpreted with confidence.