
|
GENTAUR EUROPE BELGIUM1 tel +32 2 732 5688 fax +32 2 732 4414 [email protected] Av. de l' Armée 68 B-1040 Brussels France tel 01 43 25 01 50 fax 01 43 25 01 60 9, rue Lagrange 75005 Paris Italy tel 02 36 00 65 93 fax +32 16 50 90 45 20135 Milano Germany tel +32 16 58 90 45 fax +32 16 50 90 45 Forckenbeckstraße 6 D-52074 Aachen Japan tel +81 78 386 0860 fax +81 78 306 0296 Minaatojimaminami-manchi Chuo-ku, Kobe 065-0047 |
5 Slides from 318 Euro.
The Best Tool for Therapeutic Antibody Validation
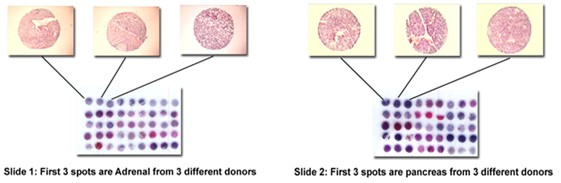
This Frozen Tissue Array is suitable for meeting US FDA requirements for Immunohistochemistry (IHC) and In vitro Diagnostic Devices (IVD) certification and also European CE Mark. This tissue array has two slides which contain 30 different human normal tissue types and 3 donors per tissue type.
NOW a 45% discount on all frozen tissue arrays from August until October 2008
Reference code: GentaurAugOct2008
Super high-quality tissues….
§ 30 different human adult normal organs according to FDA’s guideline in a set of two slides to speed up antibody validation for FDA approval
§ There are three donors per organ for better statistical result under FDA regulation.
§ Human antibody validation across 30 tissues at once with less labor and cost.
§ Gain information on expression pattern, intensity, and distribution of target proteins
Intensity of ISH Comparison between Frozen and Paraffin Sections
Applications
§ Suitable for high throughput therapeutic/diagnostic
antibody validations
§ Rapid screening of your novel gene or protein expression
against an extensive panel of tissues
§ Gene or protein expression pattern analysis
§ Comparison of expression levels of novel genes or proteins
Advantages
§ Better antigen exposure - Easier to detect by IHC and ISH
§ Less antigen modification - Better for antigen
extraction and analyses
§ High content- Save material cost and process time
§ Can be automation for Image analyses
Figure: Frozen Section vs. Paraffin Section ISH Comparison of signal intensities of hybridized VIP mRNA in mouse brain cortex on cryostat sections (A and C) and paraffin sections (B and D). Results from both 33P labeled (A and B) and digoxigenin-labeled probes (C and D) are shown. Note that intensities of hybridized signals on frozen tissue sections (arrows in A and C) are much stronger than those on the paraffin sections (arrows in B and D). -Chris Carlson at el, Optimizing In Situ Hybridization Protocols

