
|
GENTAUR EUROPE BELGIUM1 tel +32 2 732 5688 fax +32 2 732 4414 [email protected] Av. de l' Armée 68 B-1040 Brussels France tel 01 43 25 01 50 fax 01 43 25 01 60 9, rue Lagrange 75005 Paris Italy tel 02 36 00 65 93 fax +32 16 50 90 45 20135 Milano Germany tel +32 16 58 90 45 fax +32 16 50 90 45 Forckenbeckstraße 6 D-52074 Aachen Japan tel +81 78 386 0860 fax +81 78 306 0296 Minaatojimaminami-manchi Chuo-ku, Kobe 065-0047 |
NVoy Technology in Protein Circular Dichroism (CD)
Biophysical analysis of
protein solutions provides essential information about the structure and
behaviour of a
protein. Unfortunately protein instability can lead to denaturation or
aggregation, which either prevents subsequent
analysis or gives misleading data. The addition of NV10 to freshly prepared
samples can stabilise protein solutions
destined for circular dichroism spectroscopic analysis. NV10 has good
transparency in the far-UV range and does
not affect protein secondary structure, but can prolong protein stability and
inhibit aggregation. Unlike detergents,
NV10 is also compatible with analytical techniques including mass spectrometry
and so samples prepared for CD
are still suitable for subsequent analysis.
PROTOCOL
Aggregation and stability can be very protein specific, but a general protocol
is given below.
1. Determine the protein concentration (using eg.
Bradford assay, BCA assay, absorbance at 280nm).
2. Typically a fivefold excess, by mass, of NV10 will protect the target
protein. For example, use 100 μg/ml
NV10 for 20 μg/ml protein.
3. Each Stabil-P.A.C. tube contains 1.25 mg NV10 as a lyophilised powder.
4. Add the protein solution to NV10 in Stabil-P.A.C. tubes to get the desired
concentration, or make up a
2.5 mg/ml stock of NV10 (1X stock) by adding 500 μL of buffer
or distilled water to each Stabil-P.A.C.
tube and then add this stock to the protein solution.
5. This protein / NV10 solution will be suitable for near and far-UV CD
spectroscopy.
6. NV10 1X stock solution can be stored for 1 week at 4 oC or for longer term at
-20 oC.
Troubleshooting
• If the protein shows signs of aggregation or heavy
losses the NV10 to protein concentration ratio can be
increased, ie increase NV10 concentration and / or reduce
protein concentration.
• Alternatively, a lower NV10 to protein ratio can be used with proteins which
have no history of
aggregation.
• Always measure buffer blanks with buffer containing NV10.
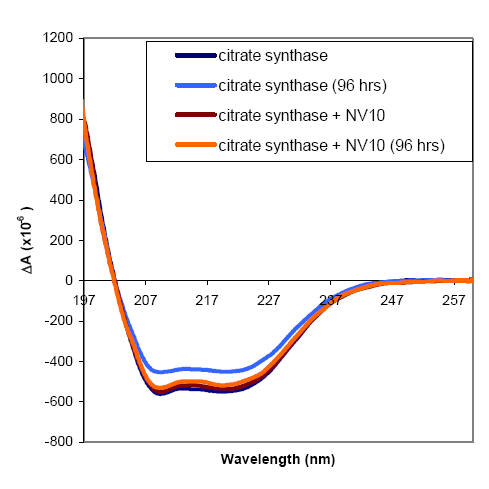
EXAMPLE : Use of NV10 in CD Analysis
Citrate synthase catalyses the first step in the
citric acid cycle. Samples stored in dilute solution at 4 oC gradually
lose activity. This is accompanied by a decrease in intensity in the far-UV CD
spectrum. The addition of NV10 to
citrate synthase samples destined for CD analysis stabilises the protein without
altering the secondary structure.
Citrate sythase was prepared at 18.6 μg/ml in either 5 mM Tris pH 7.8 alone, or
in 5 mM Tris pH 7.8 containing
186 μg/ml NV10. Far-UV CD spectra of these samples were recorded at 22 oC in a 1
cm pathlength quartz cuvette.
Buffer blanks containing either 5 mM Tris pH 7.8 alone, or 5 mM Tris pH 7.8
containing 186 μg/ml NV10 were also
recorded, and the blank spectra were subtracted from the sample spectra. The
samples were stored at 4 oC for
96 hours, then allowed to warm to room temperature and the far-UV CD spectra
were measured again.
The initial far UV CD spectrum of citrate synthase was not affected by the
presence of NV10, either by loss of
transparency at low wavelength or perturbation of secondary structure. While the
samples containing citrate
synthase alone had lost signal intensity over the storage period suggesting loss
of either material or secondary
structure, the citrate synthase protected by NV10 retained virtually the full
intensity of the far-UV CD signal. This
observation is accompanied by reduction of the enzyme activity for citrate
synthase alone, and retention of enzyme
activity for citrate synthase in the presence of NV10 after storage for 96 hours
at 4 oC.
![]()

